



The F.D.A. recommendation is likely to have a significant impact on the availability of the drugs. New York Times
Last week FDA’s ruling reclassifying narcotic pain relievers such as Vicodin and Lortab which contain hydrocodone combined with acetaminophen as schedule II drugs will make them more difficult to obtain.
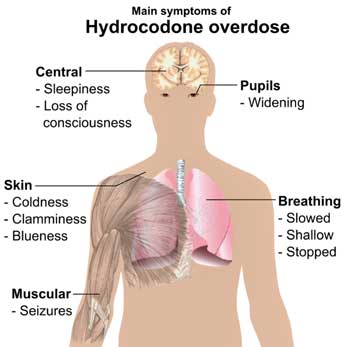
With overdoses and deaths from hydrocodone abuse skyrocketing – the FDA acted to restrict the availability of drugs some FM and ME/CFS patients rely on.
Hydrocodone is the most widely prescribed drug in the United States. With hydrocodone containing drugs making up about 70% of all narcotic pain reliever prescriptions, and approximately 137 million hydrocodone prescriptions filled in the U.S. in 2010, many people will be affected. (Eighty percent of the opioids and 99% of the worlds hydrocodone are consumed in the United States.)
The ruling reverses an era of alleged liberalization in opioid prescribing that began in the 1970’s when pharmaceutical companies argued that opioid drugs combined with acetaminophen would both reduce misuse and increase effectiveness, and should be placed in the less restrictive Schedule III category.
By the 1980’s, however, Vicodin abuse was common. The FDA repulsed DEA (Drug Enforcement Agency) requests to rein in these drugs for the past ten years, and in 2008 ruled that, while abuse was evident, the drugs were indeed misused less than opioid drugs without acetaminophen in them. Subsequent studies, however, illuminated a growing problem.
They indicated that in the past fifteen years opioid use in the US has increased dramatically. In one ten-year period (1997-2007) opioid sales increased by 150%. By 2007 the average opioid pain medication user was using four times as many opioids as in 1997.
A 2010 study found an “alarming increase” in overdose deaths mostly from oxycodone, hydrocodone, and methadone. Another study found that emergency room visits due to opioid medication abuse increased 110 percent over a four-year period (to 305,000 visits a year). A third found deaths among women due to opioid drug overdose increased five-fold over a ten-year period.
Opioid pain relievers are now believed to cause more deaths than both suicide and motor vehicle crashes. More people die from prescription drug abuse than from use of cocaine and heroin combined.
These finding set the stage for the FDA to attempt to reduce the use of these drugs markedly by reclassifying them as Schedule II drugs.
Targeting Illicit Drug Use
FDA chief Woodcock’s statement that “The FDA has become increasingly concerned about the abuse and misuse of opioid products, which have sadly reached epidemic proportions in certain parts of the United States” suggests the FDA is primarily targeting illicit use of these drugs. The agency believes the tightened restrictions will reduce the availability of these drugs to abusers, and that this need outweighs the needs of some legitimate pain drug users.
Schedule II Drug Restrictions
Prescriptions for Schedule III drugs can be refilled for as long as six months before a new prescription (and a doctors appointment) is needed.
Schedule II drugs can be prescribed for ninety days before a new prescription is required – necessitating more doctor visits and consequently increased cost. Drugstores keep Schedule II drugs in special locked areas and they require more paperwork — potentially increasing cost. Patients will also need to present their written prescriptions in person rather than calling or faxing them in -requiring more travel.
Other Schedule II drugs include morphine, oxycodone, fentanyl, Ritalin (methylphenidate), and Adderall.
American Medical Association and Other Groups Oppose Regulations
Most importantly, though, many doctors – already faced with harsh penalties linked to opioid abuse – will probably stop offering these drugs to a chronic pain populations that’s already having trouble getting help.

The American Medical Association, drug companies, patient support groups and pharmacies attempts to halt the restrictions were rebuffed.
The American Medical Association went so far as to say that the 90-day prescription restriction could “effectively eliminate the use of opioids for non-cancer pain”, and decried its effects on both doctors and patients. In an argument against a 90-day limit for prescriptions, the AMA stated
“Such a labeling change clearly would affect patients seeking medically necessary pain relief and increase the risk that prescribing physicians could be branded as practicing outside of accepted medical standards,” AMA representative.
A wide variety of groups including the pharmaceutical industry, patient support groups, physician groups (American Medical Association), and pharmacies opposed the law, but facing increasing evidence of rampant abuse in some parts of the country, and an FDA panel recommending restrictions, the FDA felt compelled to act.
The new regulations are expected to go into effect in 2014.
Some fibromyalgia and chronic fatigue syndrome patients using these drugs will likely have more difficulty obtaining them. Some may have to switch to other alternatives. Next up we’ll look at the fibromyalgia clinical trials underway to see what the future holds in this area.







I know my comment will not be liked but its how I feel.
It’s about time our government stepped in and put a stop to the ease of over prescribing of these addictive narcotics. Every time I go into the doctors about my health issues they always want to give me Vicodin for my pain. My answer every time is NO. The last thing I want is to have ME and be a drug addict.
I would rather wither in pain then become addicted to these drugs. And many times I have withered in pain and had to push through it on my own.
Our doctors would rather push narcotics on us than take the time and figure out what is wrong with us. They just want us out of their office….Next.
I’ve listened to a lot of ME sufferers who talk about wanting to get off their pain meds but the with drawls are so bad.
Just another huge reason why we need our government to push for research to find something that will help us. Because if the day comes when they find a medication to help us, a lot of people are going to have their ME symptoms gone but end up still being a drug addict.
Once you start taking these narcotics the addiction takes over and keeps telling your brain you are still in pain…take more pills. You might still be in pain but how bad is the pain really?? Your brain will tell you its unbearable and to take more pills.
Dear Readyfor life
I know its not a laughing matter but, only in the South are with drawls so bad
I have had chronic pain for 16 yrs, spinal stenosis, s/p Granuloma, osteoarthritis and I am also a disabled nurse. TRue chronic pain will not make you high. I have been on every pain med u can imagine and not once did I feel euphoria, all I felt was my pain being lessen.
I also have a Medtroinc pain pump that had to be turned off and I was operated on d/t Granuloma which is a mass inb the spine. Yes I was put on Dilaudid and Fentnayl for 3 yrs and I was sleep deprived for all that time. Now I am off them and am only on Percocet 10.
I am also a disabled nurse and am on SSI disability and will nevr work as a nurse again. Yes there are addicts that take these meds for fun, to get high but that’s not my objective. I have been in the hospital 3x for suicidal ideation d/t chronic pain.
Now I have a great dr , MRI to see if a new pain pump can even be put in with all the scar tissue. I am in pain 24/7-I get maybe 2-3 hrs a sleep a day. I can’t sleep on my bed, it’s too soft so I sleep on the couch and go back and forth between the 2.
I got all of this doing Home health Care and lifting a 300 lb patient by myself-no one else around to ask for help and my lumbar collapsed!
I only want my pump back in cause I was able to swim, walk, do normal things, etc. Thank you
Marla Jo Eshleman Winner
Oh jeez…to think it all happened lifting one huge patient!
I wish you the best of luck Maria. We’re going to be exploring new drugs and options coming up in the next blog….
Lol, @ Allan-that took me a whole minute…
Ready For Life:
What about those of us that do not abuse their drugs? That only take them for pain and as prescribed? Right now I only take Norco, but have been on oxy’s and fentanyl and xanax, I chose to get off of the other drugs. I take the min amt. I can to have a semi-so-so quality of life. I am still in pain every single day, it took me 6 years to get someone to finally listen to me about my pain. Many, many people are going to suffer, needlessly, over this.
My ex-girlfriend was on high-dose narcotic pain killers and was able to get off most of them. She was taking them like candy at one point. They weren’t working and she was having significant side effects.
However, her pain then worsened and now she’s on low-dose methadone. She takes as little as she can… She has to have something to cut the pain…. It’s a tough issue, for sure.
I agree with you – I take Vicodin every now and then when things get really bad, but manage to limit that to once a week or so…it might end up being more of a pain in the @$$ for me, but, I have also first hand know how many patients are addicted to the meds and don’t get significant relief.
So true how easy it is for a doc to write a narc prescription rather than discuss alternative pain remedies that might be more helpful in the long run, addressing cause rather than symptom. However, used appropriately, the risk for true addiction is small.
It’s sort of like McDonald’s – you know it’s bad for you and you don’t really need it, but sometimes it’s just easier to swing through the drive thru than pack a lunch or cook dinner, especially if you are really hungry. You are not addicted, but it happens to be there.
Hey readyforlife, I have rhuematoid arthritis of the spine. It’s moved into most other areas including my eyes. I’m in agony all the time.
I went through literally decades of suffering and trying other methods like NSAID’s (my stomach is now ruined) PT, massage, ice, heat..
Finally when I could no longer walk I was given pain meds. I wish you could trade places with me.
Apparently things look pretty black and white from where you are standing. This hurts the people that need the meds the most.
I used to be so active. I used to give so much to the community through volunteer work. Now I can’t walk my dog.
I suffer endlessly. This is a war on pain patients.
YOU HAVE NO CLUE WHAT YOU ARE TALKING ABOUT. THE ONLY THING I CAN FIGURE OUT IS YOU HAVE NEVER REALLY HAD ANY SEVERE PAIN AND HAD TO LIVE WITH IT DAY IN AND DAY OUT. MOST OF US WITH THESE CONDITIONS ARE NOT DRUG ADDICTS. I TRY EVERYTHING I CAN BUT AS THE OTHER POSTS HAVE SAID-SOME OF US CANNOT TAKE NSAIDS.
I ONLY TAKE VICODIN WHEN I HAVE TO AND MY DOCTOR KNOWS IT. THE GOVERNMENT STEPPING IN IS ONLY GOING MAKE IT GO UNDERGROUND AND PEOPLE WHO ARE ADDICTS WILL STILL GET WHAT THEY WANT. BUT THE ONES THAT REALLY NEED THE EFFECTIVE PAIN KILLERS WILL HAVE TO BEG THEIR DOCTORS FOR FEAR THEY WILL GET INTO TROUBLE. SO WE WILL SUFFER.
HOW UNSYMPATHETIC YOU ARE AND HOW UNINFORMED.
I HAVE TAKEN VICODIN FOR YEARS -BUT ONLY WHEN I NEED TO. YOU HAVE TO BE RESPONSIBLE AND YOUR DR CAN WITCH YOU AROUND TO ANOTHER COMPOUNDED ONE. IF YOU NEED IT.
MY ABOVE COMMENT WAS MEANT FOR READYFORLIFE!!!!!!!!
CAROLE
why make us professional diagnosed patients suffer n constant pain just because. the Government can’t control mock Doctors that use and prescribe painrelievers. for the ones that need them. I call that.not fair and not doing their job that us tax pay them to do. Jan
Dear Ready for Life; You are lucky that you can say no to more potent drugs. I expect you have yet to live the severe pain that requires a yes to more potent drugs; I also pray you never do.
FYI:
Addiction to prescription medications is not the same as the addiction to the street addict who abuses drugs.
Yes both need to be weaned off of. The difference is that when taken for medicinal purposes the addiction is physical and you do not crave the drugs while you are taking them or after you stop. When you wean off of them it takes time but you don’t battle wanting more it is your body readjusting..
There are so many drugs that require you to wean off of them for the very same reason: for example steroids, antianxiety and many more. It simply is part of the process and one that your doctor should advise you of prior to prescribing them.
Pain is subjective but I promise you if you are in severe pain and nothing is relieving it taken properly and stopped properly narcotics are safe and helpful. Sometimes you just don’t have a choice.
I hated having to take those meds and loved weaning off of them in hope never to take them again. Sadly my chronic pain is often 24/7. It steals my quality of life and ability to sleep. Chronic Pain at a 10+ all efforts and alternatives failed to bring relief so once again I had no choice. Medicinal Marijuana is not legal in my state but it would help me without the risks that come with narcotics and opiates. What choice is left? Meanwhile I continue to seek alternate solutions.
I have lost certain drugs in past because the generic was preferred and then got worse and lost three months of my life proving that I needed the brand drug. I take two brand drugs due to the way I react to the generic but other generic drugs work well for me. What will I do when they tell me I have six months to get rid of my pain and off of medication that allows me quality of life; time will tell. This is unfair to say the least.
The war on drugs has failed miserably. It’s time they tried a different approach. It’s only common sense that when they pushed harsher penalties on street drugs and put those people in prisons, that prescription medicines would become the next recreational drugs. The legal system put those people and their doctors in prison for using or prescribing prescription medicines. Heroin and other street drug deaths started to rise again, as soon as they made it more difficult to obtain prescription drugs. It’s a never ending problem. The definition of insanity is doing the same thing over and over again and expecting different results. And that’s what we have now, an insane system.
It’s a rare individual who is given Vicodin for their pain. Most do without or much less than they need to control their pain. I know. I have friends, too, who tell me how they can’t get more than “X” amount of medicines and they are clearly not enough to be of help. Some of these people are veterans of war and have injuries that cause terrible pain. Is that how we treat the men and women who have risked, and or given up their lives and their health for us? Apparently so.
We need a study that tells people how many suicides there are each year from people who simply can’t stand the pain any longer.
We need education, and rehabilitation programs for people who abuse meds. One that doesn’t make them out to be criminals so that they aren’t afraid to come forward if they need help with stopping taking them.
The fear mongering has worked too well. People who need and should have access to pain care, end of life care for instance, aren’t able to get the pain management that they need. I don’t want to see my mother or anyone suffer needlessly at the end of life, but that’s exactly what is already happening.
Also the stats aren’t telling the whole story. Anyone can make statistics to look the way they want them to. Plus autopsies are the least accurate way to determine that the cause of death when it’s from an overdose, or to tell exactly what medicines caused the death. Anyone can Google this information, if they are willing to dig a little. There are many other variables that come into play too, for instance, they “forget” to say that there was also alcohol in their system when they died from an overdose.
It’s all about making it to look the way that they want it to. Our health system has fallen apart, along with the politics that drastically effect everything our lives.
It’s a sad day when people who abuse medicines are more important than the patients who actually need them in order to have some quality of life.
There are programs for people who are addicted to medicines. Just not enough of them. In fact there were more programs available in the 80s than there are now. Why is that? It sure as hell makes me wonder….
GREAT POST. Couldn’t agree with you more.
Carole
I agree with you completely Anna. Please join me in my fight for chronic pain voices to be heard by signing these petitions. It’s part of my legal and peaceful mission in life now. I was hit by a drunk driver when I was 17. I am now 52. I have committed myself to this cause and pursue it as long as my pain permits everyday. When that drunk driver hit me my neck was broken, my pelvis broken into 8 pieces, caused swelling on my brain, cracked my left arm, cracked my jaw, broke other bones that I don’t even remember, caused internal organ damage which caused a LOT of blood loss, caused severe lesions over my left eye, caused severe bruising over most of my body. I had to learn how to walk again. I eventually had to have a total hip replacement. I suffer from chronic pain daily from these injuries. I just want chronic pain patients to be treated as legitimate patients humanely. I want doctors to be allowed to practice their chosen profession without fear of loosing their license. It’s time for our pained voices to be heard and recognized. My young life was changed instantly by someone on a legal “drug” called alcohol. I am an ex-teacher. I just want my quality of life back for the days that I do have left on this earth. I speak in support of pain patients everywhere.
http://www.change.org/petitions/congress-senate-state-officials-allow-medical-professionals-to-treat-chronic-pain-humainly#
http://petitions.moveon.org/sign/dea-vs-chrontic-pain.fb40?source=s.fb&r_by=6422065
http://www.gopetition.com/petitions/cipay-s.html?fb_action_ids=638218496222767&fb_action_types=og.recommends&fb_source=other_multiline&action_object_map=%7B%22638218496222767%22%3A10150725822361063%7D&action_type_map=%7B%22638218496222767%22%3A%22og.recommends%22%7D&action_ref_map=%5B%5D
As long as we have pain, we are going to need relief. Not everyone who needs these drugs gets addicted and not every drug is good for every person. For instance, I cannot take NSAIDS so that already limits one category of pain relievers. I get migraines from all pain meds so I am usually writhing in pain before i take a pain pill. The decision should be mine and not the government’s on what i take. The very same people that are working to rid the world of narcotic pain meds are the very same ones who testified against us at the ampligen hearings. They won’t be satisfied until the world is rid of all drugs and everyone shares in horrendous suffering. (IMHO)
The people who are taking these drugs properly are going to take a hit as the FDA clamps down on people who use them to get high. I think more than else, for me, it highlights the need for better pain drugs and new classes of pain drugs.
Drug companies are working on producing narcotic pain drugs that are less susceptible to abuse – which can’t be crushed or smoked to get high.
I’ve been taking hydrocodone for years and am certainly not addicted to it. In fact, unless my pain during the day is terrible, I only take one at bedtime, and not the highest dose either, since I can’t tolerate that much. I actually can’t remember the last time I’ve taken even two pills a day. I’ve never felt high or had a problem with the drug aside from minor side effects.
I didn’t sleep solidly through the night EVER until hydrocodone was added to my nightly meds so I’ll gladly go to my doctor every 90 days, although I think this could be a terrible burden to others who can’t afford so many doctor visits or are too ill to make that many exhausting trips.
Like Cheryl, I cannot take any NSAIDS and reacted badly to every drug prescribed for sleep too, so the fact that it also helps me sleep is a huge bonus. Thank you Anna, too for writing “It’s a sad day when people who abuse medicines are more important than the patients who actually need them in order to have some quality of life.” Amen to that!
I was in the worst pain during the first 2 years of my illness and was offered NO relief from doctors aside from the NSAIDS I couldn’t tolerate. As a result I thought about suicide far too often because the pain was so unrelenting. I’m obviously VERY dismayed by this decision.
ANOTHER TRUE POST. I NEVER TAKE VICODIN BUT AT NIGHT AND NOT EVERY NIGHT AND SOMETIMES ONLY 1/2 PILL. TRY TO SWITCH AROUND.
HOWEVER WHEN I HAD A PINCHED NERVE IN MY SPINE -MY DR. TOLD ME TO TAKE MORE AS I DID NOT WANT TO.. IT DID THE JOB AFTER 2 WEEKS.
THANKS FOR ALL THE GREAT COMMENTS.
WE ARE ADULTS AND I DO NT NEED THE GOVERNMENT TELLING ME HOW OFTEN TO TAKE A PILL.
CAROLE
Carole – could you please stop yelling? You are giving me a headache.
I cannot take NSAIDs either. They caused my kidneys to fail. I live in Stage 3 Kidney Disease now. 🙁
Please keep in mind that some people use CAPS when writing because their disability makes it difficult to read, see letters, etc. especially when tired or on certain medications. I don’t think Carole is yelling at us. She seems like a nice person. 🙂
I would like to know how many ME patients have pain. Pain is not the main symptom. I think this is another subgroup more like FM. I think autonomic symptoms are the key in this disease.
I’ve been sick for over twenty years with classic ME symptoms. The last seven years I’ve started having different types of pain. The two biggest pain issues at this time is feeling like my fingers and ribs are broken and burning pain through out my body.
My pain is for sure not my main symptom. Just another thing added to the pile of crap that I have.
Muscle pain -burning pain – after exertion has been a big problem for me. I’m not sure which is worse – fatigue or pain after exercise – it’s hard to dis-entangle the two.
I always have some pain…not the horrible pain of FM – but I definitely tend in that direction.
I am a 20 year patient with both M.E. and Fibromyalgia, I had adjusted to living well with M.E. when Fibromyalgia showed up. I would have survived non-treatment but not well and myself and my husband would have coped with the cost of non-treatment and reduction in quality of life but the cost to my children living in a home where 4 years of unsuccessful treatment meant, and the shame makes this difficult to say, periods of time where screaming could not be prevented, can not be measured.
High does treatment that is now effective has allowed me the opportunity to work with other patients and while I have seen abuse of medication and the toll that takes on patients and families I firmly believe that withholding treatment is a human rights issue. One can survive without but one can survive many things, it does not mean it is ok. For some patients at sometimes there has to be treatment and for those of you who do so well without or with little I admire and respect you. To go with out or little when our emergency rooms are seen as enemy territory by a staggering proportion of the chronic pain and illness community is much harder as you can’t get help when an increase in pain or drop in strength to face it is arises. I don’t know that one patient can understand the scope of another’s pain or ability to live with it.
I know what opiods do to my body if I had seen it as a choice I would have accepted the cost of the medication for the pay out in quality of life.
One of the better side effects of taking B1 for CFS symptoms has been a lessening of pain, CFS pain in particular, but also joint pain which had gotten pretty bad, and is now back to a dull roar.
I also take Celebrex – which is the only thing that has worked so far for the pain that goes through every muscle fiber down to finger and toe tips. It must have to do with neurotransmitters, because Celebrex is a cox2 inhibitor (whatever that means).
Pills are easier – and sometimes the only possibility – but there are methods and behavioral changes and other possibilities for some people – and they are free and have no side effects (I don’t tolerate meds well, so I had no choice but to learn about the alternatives – opiods make me too groggy to function) and always available, without prescription.
Sometimes the only thing that works is a massive amount of a proper painkiller. After back surgery I was in excruciating pain for 4 days before a doctor finally prescribed Fentanyl patches, I got the pain back under control, and got off of them before they came anywhere near to addiction, but I will tell no person how to manage their pain – which is what the FDA is doing.
Does anyone notice that they already have plenty of regulations? The correct solution is for them to enforce their current policies first, not to make draconian new ones.
Anyway, if you haven’t considered B1 as part of your ME/CFS/FM strategy, there is information on this blog, on Prohealth, and on my blog for you to think about. The best life is one without the powerful drugs to manage CHRONIC pain – where possible. Not advocating it, just adding it to the ‘possible solutions’ category.
Alicia
Alicia- What dosage of B1 are you taking and how do you take it.? Also when.
Does it have to be with other B vitiamins and can you take it at bedtime?
Thanks,
Carole
Could someone explain what the phrase “90 day prescription” means in terms of the patient?
DO NOT ASSUME THAT “ONLY” FM IS CREATING CHRONIC AND/OR ACUTE PAIN AS HAPPENED TO ME FOR YEARS. Be sure that you rule out any vertebral or disc issues, especially if your pain is primarily in only one location such as your arms or one arm… or your lower back area if the pain or numbness starts or is located at your mid-butt…. and worse yet if it then fully or in part wraps around the side of your thigh and down your lower leg.
For myself, it was FINALLY discovered that I have a degenerative disc disease which is auto-immune in nature, I believe, in addition to my ME/CFS. Ankylosing spondylothiasis. This only furthers my personal belief that it is NOT coincidental that the CFS/ME viruses continually in question reside quietly at the “basal ganglion”, the start of each nerve where it branches away from the spinal cord before going thru its vertebral outlet and then out to whichever portion of the body it innervates, both in a sensory(feeling) and motor(movement) capacity.
Anyway, in January 2012 I had 2 severely herniated, not simply “bulging”, discs removed that were taking my spinal cord along with them. But the pain in my arms remained. A second MRI done uncovered the fact the the interior of my vertebrae were calcifying (closing up) and consequently squeezing my spinal cord. in August 2012 I had a second much more invasive surgery in which the surgeons carved out the interior of 3 of my cervical vertebra thereby releasing my spinal cord and greatly improving the pain in my arms. The caveat: now I must have “pain management” due to the trauma caused during the second surgery when they had to cut away and strip back every muscle attached to the back of my neck in order to reach the interior of my neck bones.
Moral of the story: I wish I knew EXCEPT TO ENCOURAGE Y’ALL TO NOT ASSUME THAT ALL OF YOUR PAIN IS DUE TO FIBROMYALGIA and that not all of your symptoms should be continuously assumed to be secondary to CFS/ME. Feces happens such as what I just described above along with cancers, hormonal imbalances, and other physical disorders. Just keep the faith and find the laughter. And remember that you CHOOSE your physician and it is your choice as well to terminate that relationship. Hugs. marcie myers
Thanks Marcie – an important thing to keep in mind!
The ninety day prescription rule means you have to return to your doctor every ninety days (instead of every six months) in order to get your prescription refilled.
I’m very invested in this article as I take Vicodin for pain relief and have taken other narcotics as I cannot take NSAIDS anymore, it has destroyed my stomach. I’ve been with my doctor for 13 + years, tried all her suggestions, and take other, non-narcotic pills, for nerve and muscle pain. I have gone down on my own from taking Morphine AND Oxycodone, to just Vicodin. I take it as needed, that being said, I DO need it. Making it harder for people to get it legitimately I fear will only make it worse for the ones who don’t need it to overdose on it. Criminals will always find a way to get what they want, it’s us legitimate patients who will suffer.
Not everyone takes more than the doctor prescribes. The ones who up their doses for what ever the reason, uses them for “fun”, drinks on them, or mixes them with other drugs, are the ones who should not be on them. Mine still work and I have never had to move up in doses. With out them, getting out of bed with my back pain is extremely difficult. Eating is also something I could not do with out them. It is not like that every day so I don’t take them every day. Why don’t doctors just test patients to see if there is more in the patient’s system than has been prescribed? Again, those people should not be on them in the first place. Not everyone becomes addicted to them. And, it should not happen as long as you follow the doctor’s instructions. This is just going to make a whole lot of people suffer for no good reason. The same people who abuse them now, will be able to get their hands on them. And those of us who are careful and law abiding pain patients, will not be able to get the relief that every human deserves. I do also agree that maybe now the doctors will treat the original problems instead of treating just the pain symptom. I would love to be on prednazone instead of the pain pills. Maybe at least they will give us that? I don’t know. I know some people can not afford to be seen that often. I feel really bad for those patients.
Kathy
That’s the problem in a nutshell, Kathy. Most people taking these drugs for chronic pain probably want to take as few painkillers as possible and titrate down their doses as quickly when they can. My ex-girlfriend is an example; she takes her opioid pain killers as little as possible and has been off them completely at times. The responsible patients are getting caught in the middle. These drugs will still be available but they may be more difficult to get and their expense may go up a bit.
I have chronic back pain, no Ms, but I know people with MS. I am not very comfortable with this new “Crackdown” . I already have to drive over an hour to pick up my prescriptions, and wait while they verify them. I have never gotten high, or abused my meds in any way, yet I dreads even going to the pharmacy. I have had to go without meds numerous times over the years, and not jus the Opiates, because of Pharmacy problems or the script being written the wrong way.
I do not even think I would need Opiates if my condition had not been misdiagnosed for over 20 years! I had a ruptured disc , which was misdiagnosed by a (Cocaine addicted doctor). He was busted red handed years after misdiagnosing me. Not only was I misdiagnosed My Doctors wrote in their notes that I had a Psychiatric Disorder and I shouldn’t be encouraged with my “somatic” complaints. The ruptured disc caused agony for about 5 years, my leg was nearly paralyzed. My life was ruined! I had surgery where the disc was so damaged they had to remove it, without fusion. I also had a hemilaminectomey. This Doctor butchered some other patients also! He was put on desk duty but too late for me. I also have a broken tailbone, which was ignored for years, no cartilage in my neck, and an implant, which was also ignored until I had permanent nerve damage. I have bulging discs in my T-spine which hurt a lot! I have had do go on SSDI and into poverty!
I am so tired of the ignorant comments, and being punished because people abuse drugs! I can understand the mindset of the people who do commit suicide. It disgusts me that it is considered alright for people to live like this with chronic pain!
I have painful nerve ablation treatments, and sometimes they help. I have been through absolute hell. Here is just one more nightmare to deal with. My State has already implemented some of these idiotic and capricious rules. I have read that most drug abusers are turning to heroin, and that the numbers are not realistic, and that opiate abuse has dropped. I have also read that anyone with these opiates in their system that dies is labeled as a death from abuse of these medications, I am not sure if this is true, supposedly if someone dies in a car crash and they had Oxycontin in their system from three days before, this will be called a drug related death. The FDA and AMA have both used false documentation in the past and are not above it.
we should focus on the Social problems that lead to drug abuse, and suicide! Instead of the bogus “War on Drugs”!
What a tough situation Kathy. What a road you’e had. I agree on focusing on the social problems that lead to drug abuse. The feds have taken the easier way out. Good luck!
I want to say that more control is put on hydrocodone not only for the purpose of the Class 2 drug derived from codeine (an opiod) but also because of the required US FDA commercial marketing brands to be combined with another medication, in most cases this is usually acetaminophen. At this point, the drug then becomes a Class 3 drug.
The true purpose to gain more control over hydrocodone is to help with possible abusers of the drug but also to avoid issues of hepatotoxicity of acetaminophen containing formulations (or chemical-driven toxicity of the liver). In other words, sure the FDA is worried about the abuse of hydrocodone and they have multiple drug companies that were coming up with a time release form of hydrocodone only to control abuse of the drug but also to combat the issue of what was once known as Tylenol/acetaminophen overdose. This new drug is called Zogenix and the FDA just approved it on 10/25/13 and it is expected to be released in the first quarter of 2014.
I can understand your point of view, Readyforlife, due to the fact that I have worked in the health insurance industry for a major insurance company across our nation but I have also worked with a group of hospital based physicians (hospitalists) and seen the different patients coming through the ER to eventually be admitted.
I personally don’t like where our nation is going with health care and insurance coverage (but that is a completely different subject), however, I’ve seen both sides or I should say all 3 sides because I’m also a patient of fibro, restless leg syndrome, migraines, sacroiliitis, deg joint disease and not to mention I have a previous heart condition to worry with as well. I’ll be 41 in December but already live with all of this. My 86 year old grandmother passed away back in July with a very similar medical history and she had been on oxycontin (1 pill 3x’s daily) as well as too many other meds to mention but she still functioned daily.
Your lack of understanding is very upsetting to me. It’s ignorance that caused my 18 year old son to decide to listen to someone with fibro only in her early 30’s raising 2 kids alone working 40 hours a week to decide that I was lazy and needed to get “my sorry ass off the damn bed and find a job” so that his dad didn’t have to stress over money anymore. This kind of ignorance is what is about to end my 20 year marriage.
I am a Pharmacist dealing daily with people with chronic pain. It is terrible that because of the few people that abuse Vicodin the many will suffer.
The FDA should walk in the shoes of the people who are in chronic pain before they make these decisions……
Dear Gerry,
THANK YOU, as a Pharmacist, for your empathy. As you may know, there is a HUGE difference between acute pain and chronic pain requiring different approaches. Wonder if you’re seeing many people being tried on Savella. I just started it in addition to my others. I’d also noticed in a newsletter that there is a research study going on with Savella re: pain control AND cognitive function in people with ME/CFS……gotta find that 1-888 # again. I had to d/c my Cymbalta 60 BID in order to initiate the Savella. marcie myers
Oh Marcie…PLEASE research Savella before you take it. It’s one dangerous drug.
I am a sufferer of crohns disease, crohns induced arthritis, chronic kidney stones (i pass 3-4 a month), and just recently was also diagnose with fibromyalgia. I have a recurring refill of vicodin, I get 99 a month, and it usually takes me 2 months to get thru that many. I too only take them when I absolutely need them and actually turned down oxy. My dr knows i do not abuse them, he has seen me at my worst and not wanting to take anything stronger. In the many e.r. visits I have had to endure due to very large kidney stones I have been asked why I am not on something stronger…..when in the e.r. I am given dilaudid or morphine. I am not nor do I want to get addicted to anything. I take over 30 pills a day for all of my health issues and do not want to feel like I HAVE to take something. I feel that doing this to all of us out there who have serious pain issues is going to cause more being addicted to the illegal drugs that are out there and will be easier to get than a legal one. Luckily for me, as a disabled veteran, the v.a. looks out for me. I hope that when this does go through, that all the other pain sufferers like me do not suffer more than they have to.
I have many back issues and fibromyalgia. I take loratab for my pain.
I am not addicted and i have had this mess for 15 years.
Only had pain meds for three years now.I Really dont undetstand
How one gets addicted who is truly ill. My dr or insurance one only allows
Me 20 pills per month..no more..no less! So i have to manage when its necessary
To take one…now how is one to get addicted to that! Whats going to happen
Is people who are in true pain are not going to be able to get them from
Their dr and these so called addicts will still get them…from
The streets.. you can not punish good people for what bad people do.
It wont work that way.I get so tired of being punished for what others do.
Its just not right…There are better ways to regulate and stop these addicts
Without making the ones in need suffer!!,,
Hospice patients are rightfully given any and all pain meds they need to end their life with as little suffering as possible.
What about those of us who still have the gift of life but can’t enjoy or participate in it? Is there something wrong with helping us to suffer less and enjoy life more
Cort,
Thank you for this great article and the conversation it created. Thank you all for sharing your stories.
One of the real issues that many of us here have experienced is that our FM/CFS/ME label hurts us. 99.99% of our medical providers see our chronic pain as somatic or psychiatric.
Like most of the repliers here I have had a perfect storm of spinal stenosis, bulging discs, pain from facet joints and my neck surgery in 2005 (cervical discectomy with fusion)that launched my M.E. into orbit.
Like Lou Reed sang…..”You need a bus load of faith to get by”. Again, thanks to all who responded. Your replies remind me that I am not alone.
Ready for life,
My sister is a nurse anesthetist and she says the addiction rate for TRUE chronic pain sufferers is ALMOST non existent!! If you are using your Dr. prescribed pain meds for ACTUAL chronic pain the way they were prescribed to you, you are NOT GOING TO BE ADDICTED, you ARE going to be TOLERANT and DEPENDANT….that is NOT THE SAME THING and it is NOT A BAD THING….just a natural part of taking opiod pain meds for a long period of time.
I wake up in pain every single day of my life. I have fibromyalgia, Interstitial Cystitis, Endometriosis, Vulvodynia, Spinal Stenosis, Degenerative Disc Disease, Migraines, & Vitiligo, just to name the most compelling problems. I would LITERALLY not be able to function w/out pain meds. I recently lost 70 pounds w/diet and DAILY exercise, so NO, I do NOT sit around in bed EVERY DAY doing nothing….but some days I still cant function. W/out my pain meds, (OXYCONTIN and DILAUDID), I would not be able to live my life. I actually used to be on MUCH more and higher doses but I decided on my own to taper off much of my pain meds myself. My docs were AMAZED, cuz nobody ever does that!! After losing the weight and getting more fit, I just felt a LOT better….dont get me wrong, I am still in pain every day, I just feel better overall and exercise helps. I struggle to deal w/the pain cuz exercising itself is EXTREMELY PAINFUL for me and the recovery is sometimes excrutiating!! W/out my pain meds, it would be IMPOSSIBLE to exercise and maintain a healthy lifestyle!!!
The government has ABSOLUTELY NO BUSINESS IN MY PERSONAL HEALTH DECISIONS, THOSE ARE BETWEEN MY DOCTOR AND !!! If we let the govenment interfere w/our health matters, we are screwed!! Look at the clusterfuck Obamas Health Care Crap has turned out to be….YIKES!!! NO THANK YOU MR. Communist!! I already wish I was able to get medical marijuana, but the pain clinic I go to does drug testing, and if I test positive, I lose my pain meds….so thanks to our f-ing Federal Government….I can NOT get marijuana, a NATURAL drug that is scientifically proven effective in easing bladder pain and frequency, migraines, and chronic pain of ALL kinds…..and is NOT TOXIC to the body like my other pain meds are…..even tho my state of California would allow me to have a medical marijuana card and I would LOVE to try it!!! If I do try it even once to see how it works and then I get drug tested at my doctors….sayonara to my pain meds for good…and they would just cut me off cold turkey…hello w/drawals!!! Then, what if the marijuana doesnt work for me as well, or AT ALL? I have NO IDEA…..I have NEVER even tried it!!!! UGHHHH!!! See what I mean about the government and our own health decisions? My own pain doctor even said he thinks med. Marijuana would be GREAT for me….but he cant recommend I try for the reasons I listed above!!
Im out…..I am so annoyed now…..
In regard to Savella—I tried Savella 3 yrs ago and it was a horrible experience. The lowest dose caused me to go into anxiety approx. 1 hr after taking the tablet. I tried it 5 times to make sure it was really the Savella, and it happened every time. I have a friend who swears by it. I went for years taking NSAIDS and eventually had to stop due to stomach problems. I have no choice but to take a narcotic, which by the way, doesn’t make me sleepy or high. It just helps me to be able to function better. I can’t afford hired help, I have to take care of my own home, etc. Every time I do anything extra like a little yard work, I pay the next day by having to be in bed with fatigue, more pain and also depression. I have a strong constitution to be able to put up with this for so many years (27 yrs). Diagnosed with CFS in 1993, also spondylolisthesis, bulging discs, facet degeneration, sensory neuropathy both feet and legs. Had to leave my job due to fatigue same year, widowed 3 yrs ago and would love to be able to work for extra income and also the emotional well being. Putting Vicodin in the Class II category will cause me extra hardship because of the extra energy it takes to get the prescription. I fear for those who may just give up and take their life because going thru all the hassle to get pain relief is too much for them. This law will just make life more difficult for those who already have a difficult life. Chronic pain is different than acute pain—-it wears a person down, and we need a break from it. I can handle acute pain, but when it goes on and on and on for years, it makes no sense to be a martyr and suffer with it when there is a solution. Who are the people that pushed to have this law passed, anyhow?