


Dr. Pretorius’s microclot findings (with Douglas Kell) have generated a lot of interest.
What’s a potential medical breakthrough without a little controversy? Resia Pretorius Ph.D., a South African researcher from the Stellenbosch University in South Africa, and her colleague Douglas Kell from the University of Liverpool, UK, are right in the thick of things in long COVID.
Over the past ten years, Pretorius has produced over a hundred papers on clotting, platelet activation, and iron dysregulation in various diseases. She found evidence of massive platelet alterations (platelet blebbing, platelet-derived microparticles, massive fibrin networks) in lupus in 2014. Her 2016 paper highlighted “angry platelets” in diabetes. Her 2022 paper fingered platelets in Alzheimer’s and Parkinson’s disease.
During proteomic studies, she and Kell kept finding unusual deposits at the bottom of their samples – indicating something was not breaking down properly. Increased levels of inflammatory molecules, including increased levels of a clotting inhibitor (alpha-2 antiplasmin), suggested an inflammatory state was present.
“Horrible, Gunky, Dark” Microclots
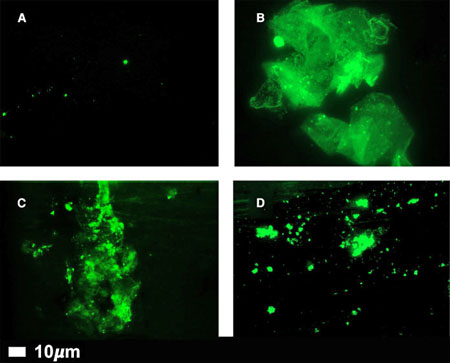
Microclots in healthy controls (A) and people with long COVID (B,C,D) (From “A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications”.)
They found strangely shaped bits of fibrin and other factors they ended up calling “fibrinaloids or microclots. Fibrin is a protein produced during the clotting process which usually forms a spaghetti-like web that binds the clot together. The fibrin they kept finding, though, was far different. According to Kell, it was “horrible, gunky, dark” stuff, “such as you might get if you half-boiled the spaghetti and let it all stick together.”
Eventually, they created a microclot grading system to keep track of them. Interestingly, different diseases appear to feature differently shaped microclots. They’ve also been finding damaged platelets and other blood vessel issues in different diseases. (The activated platelets, they believe, are interacting with the microclots and inflammatory factors to cause widespread endothelial dysfunction.)
The “microclots” they’ve found are quite variable in size but can be large enough to jam up the smallest blood vessels of the body – the microcapillaries – preventing oxygen from getting to the tissues. Their strange shapes are what appear to be impeding their breakdown.
Since 2017, she and Kell have found evidence of strange clotting issues and/or iron dysregulation in type II diabetes, rheumatoid arthritis, Parkinson’s, and Alzheimer’s. Then came COVID-19 and long COVID and things got really exciting.
If any pathogen was going to produce microclots, the SARS-CoV-2 coronavirus sure looked like it would. The spike protein the virus uses to infect cells can induce clotting, and clotting issues have loomed large in both the acute and longer cases of COVID-19.
Over the past year, the two have produced no less than five papers on microclots and other blood vessel issues in long COVID and one on the same in ME/CFS.
In February of this year, they published a paper, “A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications”, where they proposed that microclots, inflamed blood vessels, iron dysregulation, and platelet activation contribute to long COVID. Interestingly, since the body will slowly remove the fibrinaloids (amyloid microclots) over time, the key to resolving the problem may simply be stopping their formation of them.
Kell suggested at the Oxford ME Conference that the microclots, by blocking blood flows, are producing ischemic (low oxygen or hypoxic) events which turn really nasty when the blood flow returns. The sudden combination of oxygen-rich blood flowing into a hypoxic environment produces enormous amounts of free radicals and lots of localized damage (“reperfusion injury”). One could envision exercise-induced blood flows that temporarily break through the blood clot – leaving a reperfusion injury behind – and producing post-exertional malaise.
This issue alone, they believe, is enough to explain long COVID but note that the microclots also appear to be trapping other proteins in them – including antibodies – possibly setting the stage for an autoimmune reaction that showed up as the body reacts to these strangely shaped proteins. These trapped proteins were hidden from our sight and did not show up in blood analyses until the fibrinaloids themselves were assessed.
The Gist
- Over the past 10 years, Resia Pretorius Ph.D. has produced over a hundred papers on clotting, platelet activation, iron dysregulation, and other blood vessel issues in diseases ranging from rheumatoid arthritis, and Parkinson’s disease to Alzheimer’s.
- After uncovering deposits in proteomic studies that were resistant to being broken down she and her colleague Douglas Kell dug deeper. They found unusually shaped clots consisting of twisted strands of fibrin – a major component of clotting – as well as inflammatory molecules, antibodies, and other factors that had been trapped in them.
- These fibrinaloids or microclots showed up in different forms in several diseases – most recently in COVID-19 and long COVID. Over the past year, the two have produced five papers on microclots and other blood vessel issues in long COVID and one on the same in ME/CFS.
- They proposed that microclots, inflamed blood vessels, iron dysregulation, and platelet activation contribute to long COVID.
- The “microclots” they’ve found are quite variable in size but can be large enough to jam up the smallest blood vessels of the body – the microcapillaries – preventing oxygen from getting to the tissues. This could spark a reperfusion injury caused by a massive increase in free radicals when the blood returns to the area (perhaps during exercise).
- The strange shapes of the microclots may also be sparking an autoimmune reaction as the body struggles to remove them.
- Interestingly, since the body will slowly remove the fibrinaloids (amyloid microclots) over time, they believe the key to resolving the problem may simply be stopping the formation of microclots.
- So where’s the controversy? Pretorius developed a new technique to find the microclots, and her findings have yet to be validated by outside labs. Plus, evidence for a hypercoagulable state in long COVID is mixed.
- In fact, the entire coagulation issue in COVID-19 is a bit mysterious. The spike protein of the SARS-CoV-2 virus does trigger clotting, and clotting can be a serious issue in COVID-19, yet trials of clotting agents have not been helpful.
- Pretorius is improving her technique and outside studies to validate it appear to be underway. She asserts also that some coagulation tests either will not pick up indirect evidence of microclots or that the “normal ranges” found in some studies are probably not normal at all.
- Pretorius and Kell believe that using one anti-clotting agent is not sufficient and that a multi-pronged approach to the blood issues found in long COVID is necessary
- Their small preliminary, non-placebo controlled study included dual antiplatelet therapy (DAPT) (Clopidogrel 75 mg/Aspirin 75 mg) once a day, a direct oral anticoagulant (DOAC) (Apixiban) 5 mg twice a day, and a proton pump inhibitor (PPI) (e.g., pantoprazole 40 mg/day for gastric protection).
- All 24 patients in the trial reported their shortness of breath, brain fog, concentration, and forgetfulness were resolved and their fatigue was relieved over the 3-4 week trial.
- They also demonstrated enough blood vessel (endothelium) recovery to lower their microclot scores by almost two full units (7.1 → 5.2) to near-normal levels.
- We should know more in the near future as their technique to assess microclots gets upgraded and other researchers attempt to validate their results.
- Long COVID research continues to spark new possibilities for it and ME/CFS.
Controversy
So, what’s the controversy? One problem is that microclots were hidden from sight until Pretorius developed a new technique to observe them, and her findings need to be validated by outside labs before others will trust them. Plus, for all the talk of microclots in long COVID, the evidence for a hypercoagulable state in long COVID is mixed. One study that looked for coagulation issues in long COVID failed to find them, while another found some evidence of coagulation problems in only about 25% of long-COVID patients.
Some are worried that patients are jumping on the anti-coagulation bandwagon too quickly and given the serious side effects that some anticoagulants can have.
Pretorius and Kell acknowledge that their testing process needs to be automated to rule out the question of subjectivity, and their results validated.
Pretorius also asserted though, that what may appear to be normal test results may not be so normal at all. The “what’s really normal” issue has popped up numerous times over the years. One overtraining syndrome study, for instance, found all the test results fell into the “normal range” but were still significantly different from those found in the healthy controls.
Pretorius noted that relying on one common test of coagulation – the D-dimer test – is a mistake as well. Some of the long-COVID patients with high rates of microclot formation do have high D-dimer levels – yet it’s clear to her they have a coagulation problem.
This is because D-dimer tests are a composite measure of coagulation: how much fibrinogen was present, how much was converted to fibrin clots, and how much was lysed or broken down are all packed into it. High D-dimer readings can indicate that increased clotting was present but cannot tell us anything about the key issue in Pretorius’s and Kell’s minds: how many clots were strangely shaped and have become resistant to being broken down.
In fact, the entire coagulation aspect of COVID-19 has been puzzling. We know the spike protein of the coronavirus can trigger clotting, and that increased d-dimer levels and platelet activation are associated with more severe COVID-19 cases, yet both anticoagulation therapy and anti-platelet activation therapy have, to everyone’s great surprise, largely turned out to be busts. One researcher at a recent RECOVER webinar came to the conclusion that targeting inflammation – not coagulation – is going to be the key.
Pretorius and Kell, though, believe that the problem is not having the wrong therapy but not using enough of it. Because multiple problems exist (microclots, platelet activation, inflammation), they believe a multipronged treatment is needed and that’s what they recently tried in long COVID.
Multipronged Treatment Approach Works in Preliminary Trial
Their treatment regime is designed to give the body time to clear the existing microclots by preventing platelet hyperactivation and new microclots from forming. It includes dual antiplatelet therapy (DAPT) (Clopidogrel 75 mg/Aspirin 75 mg) once a day, a direct oral anticoagulant (DOAC) (Apixiban) 5 mg twice a day, and a proton pump inhibitor (PPI) (e.g., pantoprazole 40 mg/day for gastric protection).
This does not appear to be a trial for the fainthearted – the authors noted that such a regimen “must only be followed under strict and qualified medical guidance to obviate any dangers, especially hemorrhagic bleeding, and of the therapy as a whole.” Their preprint paper, “Combined triple treatment of fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) can resolve their persistent symptoms“, reported that all 24 persons with long COVID in the trial reported that their shortness of breath, brain fog, concentration, and forgetfulness were resolved and their fatigue was relieved over the 3-4 week trial.
They also demonstrated enough blood vessel (endothelium) recovery to lower their microclot scores by almost two full units (7.1 → 5.2) to near-normal levels. The trial – a preliminary one – did not include a placebo arm.
The authors concluded:
“The removal and reversal of these underlying endotheliopathies provide an important treatment option that seems to be highly efficacious, and warrants controlled clinical studies.”
The Future

Long COVID continues to spark interesting new ideas about what’s causing it and ME/CFS.
The good new is that the microclot findings have generated a lot of interest and it appears that some attempts to validate Pretorius’s approach are underway. Plus, Pretorius is upgrading her technique to remove any possibility of subjectivity. Hopefully, bigger treatment trials are underway.
The only thing better than a controversy is resolving one and the good news is that this one should be resolved – and it appears in the not-too-distant future. Whatever happens, long COVID is doing what we hoped it would do – produce new and potentially enlightening looks at long COVID, ME/CFS, and related diseases.
Update!
Eight months later the Pretorius group presents the results of a bigger clinical trial and urges that large, placebo-controlled, randomized trials be done.
- Coming up – the microclots in ME/CFS






Thanks, Cort. Another fascinating report. I’m wondering, have ME patients reported the Leiden Factor V gene variant as common? This genetic clotting issue is important to know, and perhaps a clue? Besides the clotting risk increase, it has been reported that there is a 67% increase in migraine headaches among teens, as well as other issues. Also, I wondered about B12, intrinsic factor, pernicious anemia – another DNA marker alert. While B12 injections have been tried by ME patients with varying success, have those patients found they have the variant in their DNA indicating lack of intrinsic factor for absorption? Seems like so many studies are referred to that were done before we had the ability to look at DNA and genetics, that it would be smart for research to back track a bit.
Thanks JaneB – I don’t know about that gene variant. What I’m learning is that there’s a lot packed into these blood and blood vessel issues – much more than clotting. It’s going to be interesting to see how this all unpacks.
I followed quite a few people on Twitter who have been experimenting with the use of enzymes (nattokinase, serrapeptase and lumbrokinase) who have been reporting some good results although some seem to have problems with nattokinase which I suspect is triggering an immune response.
This poster is a pharmacist who has organized the information https://twitter.com/organichemusic and posts findings.
I was put on Plavix with a baby aspirin due to some vascular issues and have noticed that I feel better although my blood tests for platelet aggregation was normal. When I do a fingerstick test, the blood seems less sticky.
There’s a ton of experimentation going on in this area in the long COVID community, that’s for sure.
I donated blood for the ME study Prof Pretorius did in the beginning of the year and recently received the results. It is weird to see that my results squarely fall within the ME mean, the first time in 35 years that a blood test shows somehing significant. So this gives me hope that there is some treatment to try. So far I however cannot find a clinician who is willing to treat me. I have a feeling that self treatment in this case is maybe not a good idea.
A lot of people are trying over the counter remedies although I suspect they are not nearly as effective as the drugs found in the study mentioned.
In viral infections, a well-functioning immune system and good blood flow and oxygen transport are of great importance and virus replication must be reduced.
Scientific publications have shown that low-frequency and radio-frequency electromagnetic fields (LF and RF EMF) of the electricity grid and of wireless communication :
• weaken our immune system, cause health problems and weaken our general condition,
• promote blood clotting via the Rouleau effect, which leads to reduced blood flow, reduced oxygen transport and increased risk of thrombosis,
For anyone who is interested, here is an interesting talk on coagulation in chronic disease done by Scott Forsgren (better health guy). His guest does list some alternatives to prescription meds.
https://www.youtube.com/watch?v=IsmZI46rRyg
Have you seen the Morley Robbins magnesium advocacy group. A large part of his theory is iron dysregulation.
Part of his protocol is to give blood to get rid of excessive iron.
Obviously magnesium is a big part of it too!
“The removal and reversal of these underlying endotheliopathies provide an important treatment option that seems to be highly efficacious, and warrants controlled clinical studies.”
Whenever I hear this kind of claims based on meager data, it makes me think of over-zealous academics or self-promoting con (I’m thinking of Judy Mitkowitz who claimed 97% efficacy and pushed xmrv as a treatment option right of the bat). Besides the lack of controls, 24 people from self-reporting registry with self-reporting result is not exactly a stellar science. They should wait till some other team replicates it with a better design before claiming efficacy or pushing RCT on this rather dangerous regiment.
Hear, hear!
Cort, is this the same as they found with ME ‘the sticky blood’ ? It was found in my blood a long time ago. I never heard of it again. I still don’t know how they tested this.
How they know you have sticky blood?
My guess is that it’s similar and different. Pretorius and Kell are finding microclots which no one was looking for back when the hypercoagulation studies were being done in ME/CFS a couple of decades ago.
Interesting research.
Its a pity they didnt consider Hdrogen Therapy for tackling the problem of iron dysregulation.
Drr Neil Nathan in his book Toxic page 274 says hydrogen is a fountain of youth and can deal with the iron issue. Google molecular hydrogen iron overload for studies.
wallace
Thanks for highlighting our work. However, these are very odd comments on ‘controversy’. The Sneller study does not measure clotting at all while the Townsend study only looks at D-dimer, which is irrelevant, as discussed in detail at https://portlandpress.com/biochemj/article/479/4/537/230829/A-central-role-for-amyloid-fibrin-microclots-in. More details at http://dbkgroup.org/longcovid/.
The D-dimer issue, I thought, was pretty well covered in the blog:
The blog was derived primarily from the NIH telebriefing, a rather lengthy article, and several of your papers – thanks for the info on the Sneller study.
I’m surprised that you don’t find any controversy in this area as the article and the NIH telebriefing – which included other experts in the field – felt that the findings were controversial given the inability of others to document direct or indirect evidence of hypercoagulation in long COVID and the need to independently verify your results.
Hopefully that will occur soon and we can get on with learning how these fascinating microclots that you’ve uncovered have come about the the impact they are having.
Also relavent here is the work of Dr Malcolm Kendrick The Clot thickens on Heart disease
I have some symptoms of Von Willebrand’s disease (not tested). Is it possible for someone whose blood perhaps clots less well than it should to still have these microclots? I am not sure I could tolerate the proposed anti-clotting drug regimen here anyway, but I’m curious if there’s enough of a difference between how blood is supposed to clot and the unusual microclots these researchers found for someone to have both issues.
I’m wondering if this could present as a red/white lacey rash? I’ve had this rash appear on my legs after the sun heated my legs (through pants, not direct sun exposure). Dermatologist referred to it as “sludge blood”. Rheumatologist ruled out Lupus and other connective tissue disorders. ME/CFS doc suggested possible Mast Cell Activation?? Could this actually be related to microclots??